
Acid gas is a mixture of hydrogen sulfide and carbon dioxide, with small amounts of hydrocarbon gases and usually water vapor (according to Science Direct).
One of the components in the flue gas produced in a coal-fired boiler is sulfur dioxide or SO2. Mitigation of SO2 release is critical to maintaining emissions within federal, state, and local limits. In a recent blog, Alex Filar discussed various flue gas desulfurization systems. These systems use a sorbent, calcium, or sodium sulfate is used to absorb SO2 from the flue gas.
The concern with SO2 emissions is producing sulfuric acid (H2SO4) when the gaseous SO2 reacts with moisture in the atmosphere, creating “acid rain”. However, by capturing the SO2 and controlling its reaction with water, high-purity sulfuric acid can be produced and sold for other industrial processes.
In one such system, a flowing bed of activated coke is used to absorb the SO2. Saturated coke is then moved to a heater, where the SO2 is forced out of the coke. The acid gas is cleaned of other impurities, then mixed with water to produce sulfuric acid. The desired concentration can be achieved by controlling the ratio of acid gas and water. The acid is then stored on-site for sale and transportation to the customer. An overview of this process is shown below.
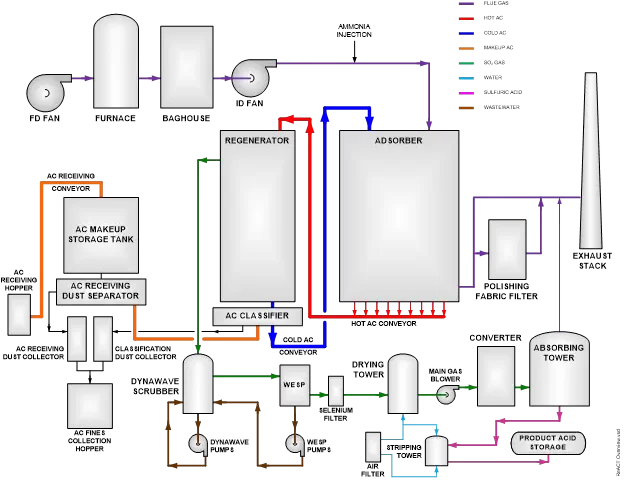
In this system, activated coke (AC) flows through the Adsorber, where it traps SO2 as well as other contaminants, such as mercury. Hot, saturated SO2 then travels to the Regenerator, where it is heated. This results in the SO2 gas being released from the AC; this allows the AC to be cooled and returned to the Adsorber to continue the process.
Over time, the movement of AC through the system (as well as the absorption process) causes the AC to break down, creating fine particles. These particles are removed in the AC Classifier; the Classifier also takes AC from the Makeup Storage Tank as needed to maintain proper AC inventory. Flue gas exits the Adsorber through a separate polishing filter to remove any AC particles carried out of the Adsorber; the cleaned flue gas is then released to the Exhaust Stack.
SO2 and other contaminant gases are released by the heating of the AC in the Regenerator. This gas is cleaned in a wet scrubber and wet electrostatic precipitator (WESP) to remove any particulate matter. It then passes through a Selenium Filter, where mercury contaminants are removed. The wet SO2 gas is dried in the Drying Tower; clean, dry SO2 gas is oxidized in the Converter to produce SO3 gas. This is then mixed with pure water in the Absorbing tower to produce sulfuric acid. Clean, high-purity sulfuric acid is then stored for sale and distribution.
Minimizing the release of contaminants into the environment is critical to the operation of any fossil-fueled power boiler. Precisely, sulfur gases must be controlled to prevent the generation of sulfuric acid in the environment. However, if the generation of sulfuric acid is done in a controlled manner, plants can both minimize the release of sulfur gases AND produce a byproduct that can be sold for use in other industrial processes.